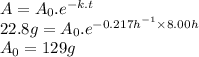Answer:
129 g
Step-by-step explanation:
The radioactive decay of a substance can be represented through the following expression.
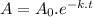
where,
A is the amount of A at certain time t
A₀ is the initial amount of A
k is the rate constant
We can find k from the half-life (t1/2) using the following expression.
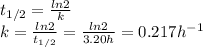
If 22.8 g of the substance is currently present, what mass A₀ was present 8.00 hours ago?