Answer:
30 grams of
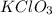
Step-by-step explanation:
The graph given in the question gives the solubility of
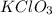 at different temperatures in water.
at different temperatures in water.
Solubility of a substance at a particular temperature is the maximum amount of it which can be dissolved in a solvent.
Saturated solution is a solution which contains the maximum amount of solute that can be dissolved in a solvent.
Hence the amount of
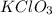 that must be dissolved at 70°C to make a saturated solution is nothing but the solubility of it at 70°C.
that must be dissolved at 70°C to make a saturated solution is nothing but the solubility of it at 70°C.
We can find the solubility from the graph for the plot of
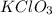 at 70°C and the corresponding value is 30 grams.
at 70°C and the corresponding value is 30 grams.