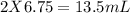Answer:
i) for NaOH (or KOH) = 13.75 mL
ii) for
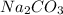 = 13.50 mL.
= 13.50 mL.
Step-by-step explanation:
Phenolphthalein is an indicator which shows change in color when the conditions are highly basic.
Methyl orange is an indicator which shows change in color in presence of highly acidic medium.
For titration of NaOH and HCl we can use phenolphthalein but for sodium carbonate (a weak base) with HCl we use mehtyl orange.
Now in case of mixture of given strong base and weak base the reading or end point obtained from phenolphthalein, shows the neutralization of NaOH only and half neutralization of sodium carbonate.


While the reading or end point of methyl orange shows the neutralization of both the base present.
a) The volume of HCl used for phenolphthalein end point = 20.50 mL
Let us say the volume of HCl used for NaOH = V1
The volume of HCl used for half neutralization of sodium carbonate = V2
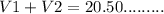 (1)
(1)
b) the volume of HCl used for methyl organe end point = 27.25
This volume of HCl used for both NaOH and Na₂CO₃
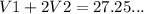 (2)
(2)
Equating equation 1 and 2
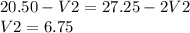
i) Thus the volume of Acid used for NaOH (Or KOH if present in place of NaOH) = 20.50-6.75= 13.75 mL
ii) the volume of acid used for sodium carbonate =