Answer:
We will use ideal gas law to find the temperature inside the balloon.
The temperature inside the balloon is 0.002564 K.
Step-by-step explanation:
We will use ideal gas law , given as :
PV = nRT
where,
P = Pressure of gas
V = Volume of gas
n = number of moles of gas
R = Gas constant = 0.0821 L.atm/mol.K
T = Temperature of gas
So,using ideal gas equation to find the temperature inside the balloon:
PV = nRT
where,
P = 20 mmHg =
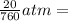
V = 10 mL = 0.010 L
n = =
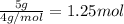
R = 0.0821 L.atm/mol.K
T = Temperature of helium gas = ?
Putting values in above equation, we get:
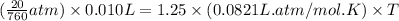
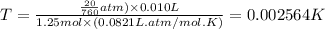
The temperature inside the balloon is 0.002564 K.