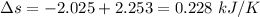Answer:
Equilibrium Temperature is 382.71 K
Total entropy is 0.228 kJ/K
Solution:
As per the question:
Mass of the Aluminium block, M = 28 kg
Initial temperature of aluminium,
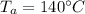 = 273 + 140 = 413 K
= 273 + 140 = 413 K
Mass of Iron block, m = 36 kg
Temperature for iron block,
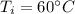 = 273 + 60 = 333 K
= 273 + 60 = 333 K
At 400 k
Specific heat of Aluminium,
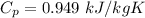
At room temperature
Specific heat of iron,
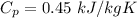
Now,
To calculate the final equilibrium temperature:
Amount of heat loss by Aluminium = Amount of heat gain by Iron
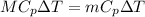
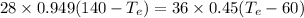
Thus
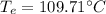 = 273 + 109.71 = 382.71 K
= 273 + 109.71 = 382.71 K
where
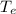 = Equilibrium temperature
= Equilibrium temperature
Now,
To calculate the changer in entropy:
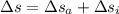
Now,
For Aluminium:
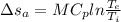
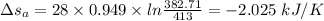
For Iron:
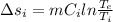
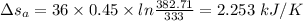
Thus