Answer: This is a single displacement reaction and the products are zinc chloride and hydrogen gas.
Step-by-step explanation:
Single displacement reaction is defined as the reaction in which more reactive element displaces a less reactive element from its chemical reaction.
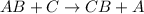
Element C is more reactive than element A.
Double displacement reaction is defined as the reaction in which exchange of ions takes place.
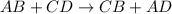
Synthesis reaction is defined as the reaction in which smaller substances combine in their elemental state to form a larger substance.
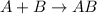
Decomposition reaction is defined as the reaction in which a large substance breaks down into smaller substances.
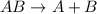
For the given chemical reaction:
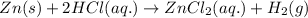
The reaction is considered as single displacement reaction.
By Stoichiometry of the reaction:
1 mole of zinc metal reacts with 2 moles of hydrochloric acid to produce 1 mole of zinc chloride and 1 mole of hydrogen gas.
Hence, this is a single displacement reaction and the products are zinc chloride and hydrogen gas.