To solve this problem it is necessary to apply the concept related to root mean square velocity, which can be expressed as
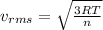
Where,
T = Temperature
R = Gas ideal constant
n = Number of moles in grams.
Our values are given as
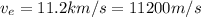
The temperature is
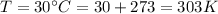
Therefore the root mean square velocity would be
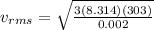
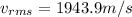
The fraction of velocity then can be calculated between the escape velocity and the root mean square velocity
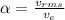
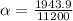
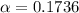
Therefore the fraction of the scape velocity on the earth for molecula hydrogen is 0.1736