Answer:
The temperature will change and become 2/3 of its original.
Step-by-step explanation:
Using Ideal gas equation for same mole of gas as
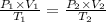
Given ,
The volume of the sample gets reduced 1/3 of the original. So,
V₂ = 1/3V₁
The pressure of the sample is doubled of the original. So,
P₂ = 2P₁
Using above equation as:
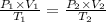
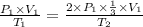
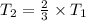
The temperature will change and become 2/3 of its original.