Step-by-step explanation:
Given that,
The disintegration constant of the nuclide,
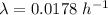
(a) The half life of this nuclide is given by :
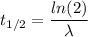
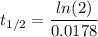
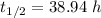
(b) The decay equation of any radioactive nuclide is given by :
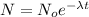
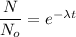
Number of remaining sample in 4.44 half lives is :
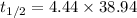
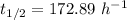
So,
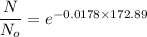
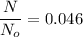
(c) Number of remaining sample in 14.6 days is :
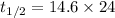
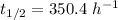
So,
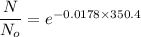
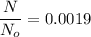
Hence, this is the required solution.