The given question is incomplete. The complete question is:
In the reaction
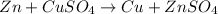 , identify the oxidizing and reducing agent.
, identify the oxidizing and reducing agent.
Answer: reducing agent. : Zn
oxidizing agent :
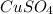
Explanation:
Oxidation reaction : When there is an increase in oxidation state number.
Reduction reaction : when there is a decrease in oxidation state number.
Zinc metal has undergone oxidation, as its oxidation state is changing from 0 to 2+. Zinc metal is getting converted into zinc ions.
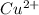 ions has undergone reduction, as its oxidation state is changing from +2 to 0. Copper ions are getting converted into copper metal.
ions has undergone reduction, as its oxidation state is changing from +2 to 0. Copper ions are getting converted into copper metal.
The chemical agent which itself get oxidized and reduce others is called reducing agent.
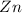 is a reducing agent.
is a reducing agent.
The chemical agent which itself get reduced and oxidize others is called oxidizing agent.
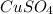 is an oxidizing agent.
is an oxidizing agent.