Answer:
The total energy required is 121.56 kJ
Step-by-step explanation:
The energy required to heat a m = 40g sample of ice is calculated using the following information:
The specific heat of ice =
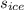 = 2.108 J/g-K
= 2.108 J/g-K
The specific heat of water =
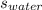 = 4.187 J/g-K
= 4.187 J/g-K
The specific heat of steam =
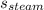 = 1.996 J/g-K
= 1.996 J/g-K
The latent heat of fusion for ice = L = 336 kJ/kg
The latent heat of vaporisation = l = 2260 kJ/kg
The latent heat of fusion is the amount of energy required to change one kg of ice into water without a change in temperature.
Firstly, we find the energy required to change the temperature of 40 g of ice from -10°C to 0°C.
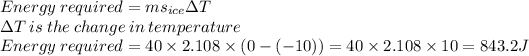
Then, the energy required to convert 40g of ice at 0°C to water at 0°C.
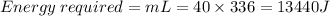
Then, the energy required to convert water at 0°C to water at 100°C.

The energy required to convert water at 100°C to steam at 100°C.
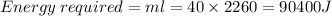
The energy required to convert steam at 100°C to steam at 105°C.
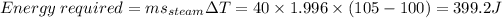
Total Energy required = 843.2 + 13440 + 16748 + 90400 + 399.2 = 121560.4J = 121.56 kJ