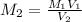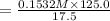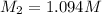Answer:
Step-by-step explanation:
Given data:
V1 = 25.0 ml
M2 = 0.00383 M
V2 = 1000 ml
we knwo that
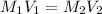
∴
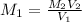
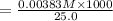
= 0.1532 M
0.1532 M is concentration in 25 ml but taken from 125 ml solution.
∴ The concentration in 125.0 ml solution is = 0.1532 M
M1 = 0.1532 M
V1 = 125.0ml and V2 = 17.5.0ml