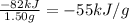Answer:
Hydrogen: -141 kJ/g
Methane: -55kJ/g
The energy released per gram of hydrogen in its combustion is higher than the energy released per gram of methane in its combustion.
Step-by-step explanation:
According to the law of conservation of the energy, the sum of the heat released by the combustion and the heat absorbed by the bomb calorimeter is zero.
Qc + Qb = 0
Qc = -Qb [1]
We can calculate the heat absorbed by the bomb calorimeter using the following expression.
Q = C . ΔT
where,
C is the heat capacity
ΔT is the change in the temperature
Hydrogen
Qc = -Qb = -C . ΔT = -(11.3 kJ/°C) . (14.3°C) = -162 kJ
The heat released per gram of hydrogen is:
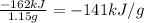
Methane
Qc = -Qb = -C . ΔT = -(11.3 kJ/°C) . (7.3°C) = -82 kJ
The heat released per gram of methane is: