Answer:
The formula for Tricarbon Octahydride is
 .
.
Step-by-step explanation:
The given question says that tricarbon means there are 3 carbon atoms in the compound and octahydride means there are 8 hydrogen atoms. Here octa stands for 8 and hydride stands for hydrogen.
Here the number of hydrogen atom is the sum of two times of number of carbon atom and of two. That is it fits in the general formula of alkane group.
General formula for alkane group =
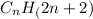 .
.
Where n stands for number of carbon atoms.
Therefore formula is
 .
.
Hence the formula for Tricarbon Octahydride is
 and this is propane.
and this is propane.