Answer:
300 nm
Step-by-step explanation:
R = Gas constant = 8.314 J/molK
r = Atomic radii =
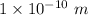
d = Atomic diameter =
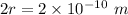
At STP
T = Temperature = 273.15 K
P = Pressure = 100 kPa
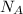 = Avogadro's number =
= Avogadro's number =

The mean free path is given by
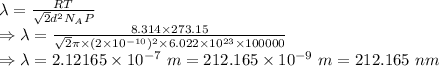
The answer that best represents the mean free path for gas molecules is 300 nm