Answer:
the amount of heat that gets through both the wires will be same.
Step-by-step explanation:
By the Fourier's law of conduction we have:
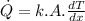
where:
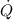 = rate of heat transfer
= rate of heat transfer
k = thermal conductivity of the material
A = area of the material
dT = temperature difference across the length dx
According to the question, the system to be analysed is isolated from the surrounding.
Until the thermal equilibrium is established between aluminium and copper wires the amount of heat that gets through both the wires will be same.
But the rate of heat transfer through the aluminium will be greater as it has double the thermal conductivity of copper.