Answer:
Given:
The pH of solution A = 2.4
The pH of solution B = 9.4.
Solution:
The hydrogen ion concentration is the amount of hydrogen ions present in the given solution usually expressed in terms of moles pere litre .
(A) hydrogen-ion concentrations of the solution.
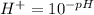
Now,
The hydrogen ion concentration of the solution A
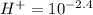
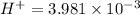
Similarly
hydrogen ion concentration of the solution B
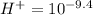
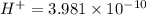
(b) The hydrogen ion concentration of solution A
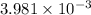 is and the solution B is
is and the solution B is

so we can conclude that the hydrogen ion concentration of solution A is
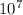 times greater than solution B
times greater than solution B
(c) The solutions A and Solution B differ by 7 order of magnitude