Answer:
D is the correct option.
Step-by-step explanation:
- The structure of ethene/ethyelene is :
 C==C
C==C

The carbon atoms are bonded together by a double bond.
- The structure of polyethylene/polythene is: (-
 C-C
C-C
 -
-
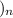
The carbon atoms are bonded together by single bonds.
Lets analyse each option:
A) Its false. Lets look at the obvious: Ethylene is a gas and polyethylene is a solid. Monomers and polymers have very different properties.
B) Its false. Polyethylene doesnt have double bonds.
C) False as well. Polyethylene doesnt have double bonds.
D) True. It is evident from the structure of the the monomer and polymer that I've shown above. I'll also provide neat structures in the attachments.