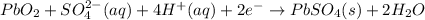Answer:
The correct answer is option e.
Step-by-step explanation:
The lead acid battery consists lead as an anode and lead oxide as a cathode. Both the electrodes are suspended in dilute sulfuric acid which act as an electrolyte.
At anode: Oxidation
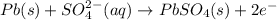
At cathode: Reduction