Answer:
A. balanced
Step-by-step explanation:
Hello,
In this case, redox reactions undergo when one the elements at the reactants suffers an increase in its oxidation state and other elements suffers a decrease in its oxidation state. Thus, one could consider the following example:
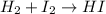
In that case, both hydrogen and iodine have zero as their oxidation states at the reactants whereas they go +1 and -1 respectively at the hydroiodic acid as shown below:
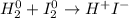
In such a way, each hydrogen increases by 1 electron (two electrons in total as there two hydrogens) and iodine decreases by 1 electron (two electrons in total as there two iodines). Thus, the balance turns out:
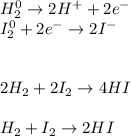
Therefore, redox equations are A. balanced when the total increase in oxidation numbers equals the total decrease in oxidation.
Best regards.