To develop this problem it is necessary to apply the concepts related to Continuity.
The continuity equation could be defined as
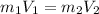
Where,
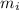 = Mass
= Mass
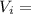 Volume
Volume
Our values are given as
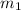 = 13.5M
= 13.5M
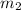 = 5M
= 5M
 = 58.0mL
= 58.0mL
Using the previous equation and rearrange to find
 we have,
we have,
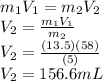
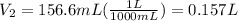
Therefore the final volume in liters would be 0.157L.