Answer:44.7 hours.
Step-by-step explanation:
The time taken for the radioactivity of a specified isotope to fall to half its original value is called half life of that radioactive material.
Half life of sodium-24 is
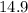 hours.
hours.
Initial amount of sodium-24 is
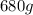
In
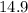 hours,the quantity falls to
hours,the quantity falls to
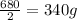
In another
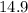 hours,the quantity falls to
hours,the quantity falls to
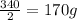
In another
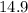 hours,the quantity falls to
hours,the quantity falls to
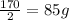
So,it takes
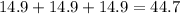 hours for the quantity to become
hours for the quantity to become
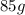 .
.