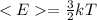Answer:
The Temperature is the measure of Average Kinetic Energy of the molecule in the substance.
Temperature and Kinetic Energy of molecules are related by the equation
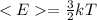
Where
<E> denotes average kinetic energy
k - Boltzmann constant
T - Temperature of the substance
Step-by-step explanation:
The energy inside the substance is characterised by translational and vibrational motion.
The molecules in a substance have a range of kinetic energies because they don't all move at the same speed. As a substance absorbs heat the particles move faster so the average kinetic energy and therefore the temperature increases.
As the molecules of a substance vibrate faster their temperature increases.
Thus an increase energy corresponds to an increase in temperature
<E> α T (α denotes proportionality)