To develop the problem it is necessary to apply the concepts related to the ideal gas law, mass flow rate and total enthalpy.
The gas ideal law is given as,
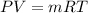
Where,
P = Pressure
V = Volume
m = mass
R = Gas Constant
T = Temperature
Our data are given by
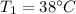
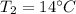
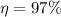
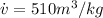
Note that the pressure to 38°C is 0.06626 bar
PART A) Using the ideal gas equation to calculate the mass flow,
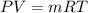
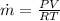
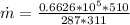
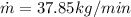
Therfore the mass flow rate at which water condenses, then
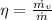
Re-arrange to find
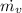
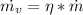
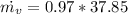
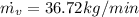
PART B) Enthalpy is given by definition as,
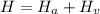
Where,
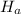 = Enthalpy of dry air
= Enthalpy of dry air
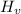 = Enthalpy of water vapor
= Enthalpy of water vapor
Replacing with our values we have that
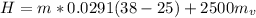
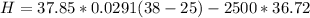
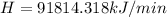
In the conversion system 1 ton is equal to 210kJ / min
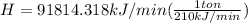
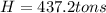
The cooling requeriment in tons of cooling is 437.2.