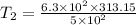Answer:394.569K
Step-by-step explanation:
Gay-Lussac's law states that at constant volume,the pressure of a gas is directly proportional to its temperature.
The pressure,temperature at any states with constant volume can be related as
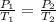
where
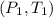 are pressure and temperature at initial state and
are pressure and temperature at initial state and
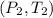 are pressure and temperature at final state.
are pressure and temperature at final state.
Given,
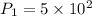
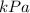
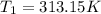
Given,
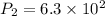
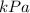
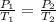
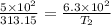
So,