Answer:
q Low = 355.95 kJ/kg
q High = 889.9 kJ/kg
net cycle work = 533.9 kJ/kg
Step-by-step explanation:
given data
efficiency = 60%
temperature = 300 K
pressure changes = 90 kPa to 120 kPa
to find out
high and low temperature heat transfers and the net cycle work
solution
we know here that Carnot cycle efficiency is the maximum possible efficiency of the heat engines system between the specific temperature limit
so that efficiency we find here as high temperature is
efficiency =
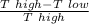 ................1
................1
we get
efficiency = 1 -
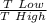 .................2
.................2
put here value we get T high that is
0.6 = 1 -
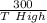
T high = 750 K
and now we get volume ratio for the heat transfer
t3 = T4 = T Low
so we get equation here as
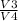 =
=
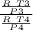 .........................3
.........................3
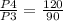 = 1.33
= 1.33
we know that value of R from A5 Table that is
R = 4.1243
so efficiency will be here
q Low = R ×T Low × ln
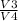 .................4
.................4
put the value we get
q Low = 4.1243 × 300 × 1.33
q Low = 355.95 kJ/kg
and
q High =
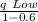 ...................5
...................5
q High =
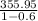
q High = 889.9 kJ/kg
so we can say that net cycle work = net transfer
net cycle work = q High - q Low .....................6
net cycle work = 889.9 - 355.95
net cycle work = 533.9 kJ/kg