Answer: The value of enthalpy change when 6 moles of NaOH is produced is -1106.4 kJ
Step-by-step explanation:
We are given:
Moles of NaOH produced = 6.00 moles
For the given chemical equation:
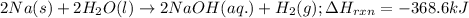
The enthalpy change of the reaction = -368.6 kJ
To calculate the amount of enthalpy change, we use unitary method:
When 2 moles of NaOH is produced, the enthalpy change of the reaction is -368.6 kJ
So, when 6 moles of NaOH is produced, the enthalpy change of the reaction will be =
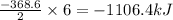
Hence, the value of enthalpy change when 6 moles of NaOH is produced is -1106.4 kJ