Answer:
Concentration of sodium carbonate in the solution before the addition of HCl is 0.004881 mol/L.
Step-by-step explanation:
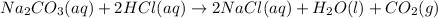
Molarity of HCl solution = 0.1174 M
Volume of HCl solution = 83.15 mL = 0.08315 L
Moles of HCl = n
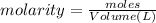
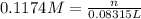
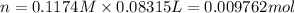
According to reaction , 2 moles of HCl reacts with 1 mole of sodium carbonate.
Then 0.009762 mol of HCl will recat with:
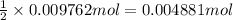
Moles of Sodium carbonate = 0.004881 mol
Volume of the sodium carbonate containing solution taken = 1L
Concentration of sodium carbonate in the solution before the addition of HCl:
![[Na_2CO_3]=(0.004881 mol)/(1 L)=0.004881 mol/L](https://img.qammunity.org/2020/formulas/chemistry/college/82s0youcg9h5nn2vjolzms6qi1lh2odmtu.png)