Answer:
T=138 °C
Step-by-step explanation:
Given that
m = 0.028 kg
Net work output W= 60 KJ
T₂=2T₁
As we know that efficiency of Carnot heat engine given as
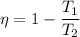
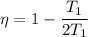
η = 0.5
We know that
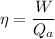
Qa=heat addition
W= net work output
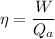
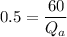
Qa= 120 KJ
From first law
Qa= W+ Qr
Qr= 120 - 60
Qr= 60 KJ
Qr Is the heat rejection.
Heat rejection per unit mass
Qr=60 / 0.028 = 2142.85 KJ/kg
Qr= 2142.85 KJ/kg
Temperature at which latent heat of steam is 2142.85 KJ/kg will be our answer.
T=138 °C
The temperature corresponding to 2142.85 KJ/kg will be 138 °C.
T=138 °C