Answer:
Q1 = 1250 kJ
Q2 = 750 kJ
temperature = 610.8°C
Step-by-step explanation:
given data
heat source temperature = 1200°C = 1473 K
thermal efficiency η = 40 percent = 0.4
maximum work ( ω max )= 500 kJ
to find out
heat supplied to the heat engine by the heat source, the heat rejected to the heat sink, and the temperature of heat sink
solution
we know that efficiency is express as
η =
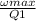 .........1
.........1
here Q1 is heat supplied and η is efficiency and ω is work
so Q1 =
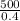
Q1 = 1250 kJ
so
ω max = Q1 - Q2
and heat rejected to heat sink Q2 is
Q2 = Q1 - ω max
Q2 = 1250 - 500
Q2 = 750 kJ
and'we also know that here
efficiency η = 1 -
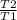
0.4 = 1 -
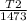
T2 = 883.8 K
temperature = 610.8°C