Answer :
(a) The mole fraction of aniline in the mixture is 0.345
(b) The relative amounts of the two phases is 0.136
Explanation :
(a) First we have to calculate the moles of aniline and hexane.
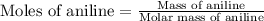
Molar mass of aniline = 93.13 g/mole
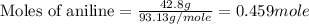
and,
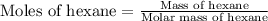
Molar mass of hexane = 86.18 g/mole
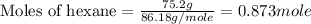
Now we have to calculate the mole fraction of aniline.
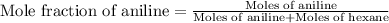
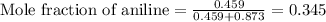
Thus, the mole fraction of aniline in the mixture is 0.345
(b) Applying Lever rule, the ratio of amount of each phase:
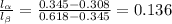
Thus, the relative amounts of the two phases is 0.136