Answer:
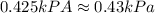
Step-by-step explanation:
In order to solve the problem we must resort to Taylor's approximations in which it is possible to obtain an approximation through a polynomial function.
For the particular case we proceed to make a linear approach.
Our values are defined as,
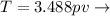 Relation of temperature, pressure and volume
Relation of temperature, pressure and volume
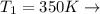 Initial Temperature
Initial Temperature
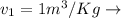 Specific Volume
Specific Volume
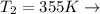 Final Temperature
Final Temperature
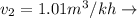 Final Specific Volume
Final Specific Volume
The previous equation can be expressed as function of pressure, i.e,
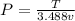
We can differentiate the expression in function of temperature and the specific volume, then
Temperature:
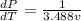
Volume
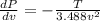
PART A) Then the total change of the pressure is given by,
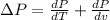

Replacing the values given, we have
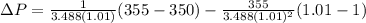
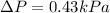
PART B) Now we can calculate the exact change in pressure through the general equation, that is
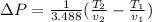
Replacing the values we have:
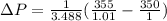
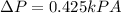
We can conclude that the approximation by Taylor's theorem is close to the value calculated by the general expression.