Answer:
33.5 kJ
Step-by-step explanation:
here there is no difference is made in the temperature. Only thing happens here is the conversion of the ice in to water of 0 degree. The heat energy taken from the outside is spent for this conversion.
we have ice 100g =0.1 kg
Appplying Q=mL
Q=
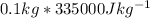
Q = 33 500 J
Q = 33.5 kJ