Answer:
Not possible.
Step-by-step explanation:
According to second law of thermodynamics, the maximum efficiency any heat engine could achieve is Carnot Efficiency η defined by:
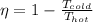
Where
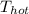 and
and
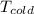 are temperature (in Kelvin) of heat source and heatsink respectively
are temperature (in Kelvin) of heat source and heatsink respectively
In our case (I will be using K = 273+°C) :
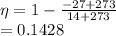
In percentage, this is 14.28% efficiency, which is the maximum theoretical efficiency any heat engine could have while working between -27 and 14 °C temperature. Any claim of more efficient heat engine between these 2 temperature are violates the second law of thermodynamics. Therefore, the claim must be false.