Answer:
a) 2.89x10⁶ kg
b) 402 kg/s
Step-by-step explanation:
Let's assume that the power plant operates steadily (without changing in the state) and that the kinetic and the potential energy changes are zero.
a) The rate of the amount of heat inputs to the power plant, is the net power divided by the thermal efficiency (32% = 0.32):
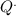 = 300/0.32
= 300/0.32
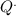 = 937.5 MW
= 937.5 MW
The rate is the amount of heat divided by the time, so the amount of heat is the rate multiplied by the time (24h = 86,400s):
Q = 937.5 * 86,400
Q = 8.1x10⁷ MJ
The mass of the coal is the heat divided by its heating value (28,000 kJ/kg = 28 MJ/kg)
m = 8.1x10⁷/28
m = 2.89x10⁶ kg
b) The rate of air/fuel is 12 kg/kg, so the mass of air (ma) is:
ma/m = 12
ma/2.89x10⁶ = 12
ma = 3.47x10⁷ kg
During 24 h(86,400s) the rate of air is:
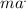 = 3.47x10⁷/86,400s
= 3.47x10⁷/86,400s
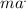 = 402 kg/s
= 402 kg/s