Answer:
Here's what I get
Step-by-step explanation:
If the orbital is 5d, n = 5 and l=2.
mₗ = -l, -l + 1, -l + 2, …., l
(mₛ is a quantum number for electrons)
Thus, we can have the following combinations.
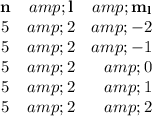
That makes a total of five 5d orbitals.
The pictures below show what two of them look like.