Answer:
The answer is:
P/m = 612.97 kJ/kg
Δs = 0.2566 kJ/kgK
Step-by-step explanation:
In this case we have the following values and calculate:
STEP A:
water --> p₁ = 1 MPa and T₁ = 200 °C
saturated liquid and vapor --> T₂ = 40 °C
h₂ = h₁(40)+x₂(hv(40)-h₁(40))
h₂ = 167.57+0.83*(2574.30-167.57) = 2165.13 kJ/kg
s₂ = s₁(40)+x₂(sv(40)-s₁(40))
s₂ = 0.5725+0.83*(8.2570-0.5725) = 6.9506 kJ/kgK
STEP B:
Now we have all of the values required for making calculations. But we now the power is required, too
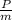 = h₁-h₂
= h₁-h₂
= 2827.9-2165.13
= 612.97 kJ/kg
Δs = 0.2566 kJ/kgK