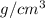Answer: 2.9
Step-by-step explanation:
from the question we are given the following:
mass of rock = 220 g
volume of displaced water = 75

the density of an object is gotten from the formula density =

From the question, we were given the mass of a rock and volume of water the rock displaces.
When water is placed in a graduated cylinder and a rock is placed in the water, the volume of water the rock displaces is the increase in the reading of water after the rock has been put in it. The amount of water displaced is known as the volume of the rock.
therefore volume of the rock = volume of water displaced
density =
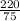 = 2.9
= 2.9