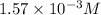Answer: The concentration of carbon dioxide in air is
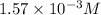
Step-by-step explanation:
We are given:
4.0 % carbon dioxide by volume
This means that 4.00 mL of carbon dioxide is present in 100 mL of solution
To calculate the amount of carbon dioxide, we use the equation given by ideal gas which follows:

where,
P = pressure of the gas = 1 atm
V = Volume of the gas = 4.0 mL = 0.004 L
T = Temperature of the gas =
![37^oC=[37+273]K=310K](https://img.qammunity.org/2020/formulas/chemistry/college/g4qi44srgtaq7fmv4dze4d1dxww2knro75.png)
R = Gas constant =
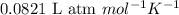
n = number of moles of carbon dioxide gas = ?
Putting values in above equation, we get:
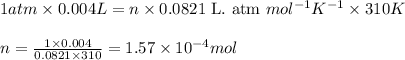
To calculate the molarity of solution, we use the equation:
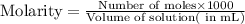
Moles of carbon dioxide gas =
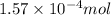
Volume of solution = 100 mL
Putting values in above equation, we get:
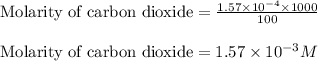
Hence, the concentration of carbon dioxide in air is