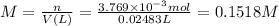Answer:
0.1518M
Step-by-step explanation:
Let's consider the balanced complete chemical equation for this double displacement reaction.
Fe(HSO₄)₂(aq) + Na₂CrO₄(aq) ⇄ 2 NaHSO₄(aq) + FeCrO₄(s)
We can write it as a full ionic equation.
Fe²⁺(aq) + HSO₄⁻(aq) + 2 Na⁺(aq) + CrO₄²⁻(aq) ⇄ 2 Na⁺(aq) + HSO₄⁻(aq) + FeCrO₄(s)
We can also write the net ionic equation.
Fe²⁺(aq) + CrO₄²⁻(aq) ⇄ FeCrO₄(s)
We know the following relations:
- The molar mass of Fe(HSO₄)₂ is 249.99 g/mol.
- 1 mole of Fe(HSO₄)₂ reacts with 1 mole of Na₂CrO₄
Then, for 0.9422 grams of iron(II) bisulfate:
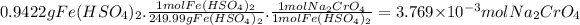
The molarity of the sodium chromate solution is: