Answer:
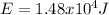
Step-by-step explanation:
Hello!
In this case, since the energy implied in a heating process is computed by using the following equation:
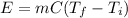
Whereas m is the mass, C the specific heat and T the temperature. In such a way, by plugging in the given mass, specific heat and temperatures, we obtain the following energy:
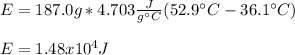
Considering that the specific heat can by used by unit of °C or K because their difference is equivalent.
Regards!