Answer:
There are present 5,5668 moles of water per mole of CuSO₄.
Step-by-step explanation:
The mass of CuSO₄ anhydrous is:
23,403g - 22,652g = 0,751g.
mass of crucible+lid+CuSO₄ - mass of crucible+lid
As molar mass of CuSO₄ is 159,609g/mol. The moles are:
0,751g ×
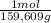 = 4,7052x10⁻³ moles CuSO₄
= 4,7052x10⁻³ moles CuSO₄
Now, the mass of water present in the initial sample is:
23,875g - 0,751g - 22,652g = 0,472g.
mass of crucible+lid+CuSO₄hydrate - CuSO₄ - mass of crucible+lid
As molar mass of H₂O is 18,02g/mol. The moles are:
0,472g ×
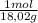 = 2,6193x10⁻² moles H₂O
= 2,6193x10⁻² moles H₂O
The ratio of moles H₂O:CuSO₄ is:
2,6193x10⁻² moles H₂O / 4,7052x10⁻³ moles CuSO₄ = 5,5668
That means that you have 5,5668 moles of water per mole of CuSO₄.
I hope it helps!