Answer:
The enthalpy of the reaction is 18.616 kJ/mol.
Step-by-step explanation:
Heat released by the solution = Q
Mass of the solution = 25.0 g
Heat capacity of the solution = c = 4.20 J/g K
Initial temperature of the solution ,
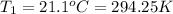
Final temperature of the solution,
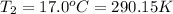
Change in temperature of the solution = ΔT =
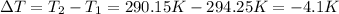
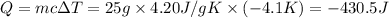
Heat gained during the reaction of ammonium nitrate = Q'
Q' = -Q (energy remained conserved)
Q'= -(-430.5 J)=430.5 J
Mass of ammonium nitrate added in water = 1.85 g
Moles of ammonium nitrate added in water =
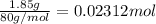
0.02312 moles of ammonium nitrate absorbs 430.5 Joules of energy. the 1 mole will absorb:
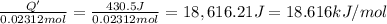
The enthalpy of the reaction is 18.616 kJ/mol.