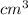The volume of the gas when the pressure is
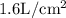 is 200
is 200
Solution:
Given, the volume of a gas in cubic centimeters, V, varies inversely with the pressure of the gas in liters per square centimeter, p.
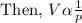
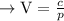
where "c" is proportionality constant.
Given that when the volume of the gas is 16
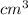 , its pressure is 20
, its pressure is 20
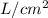
So, substitute the above values in our formula
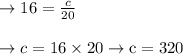
Now let us find the volume of the gas when the pressure is
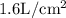
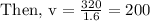
Hence, the volume of the gas is 200