Answer:
145 grams of a 0.480 % KCN solution contains 697 mg of KCN.
Step-by-step explanation:
w/w % : The percentage mass or fraction is mass of the of solute present in 100 grams of the solution.
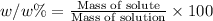
given = w/w% = 0.480 %
Mass of solute that KCN = 697 mg = 0.697 g
(1 mg = 0.001 g)
Mass of the solution = ? = x
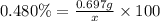
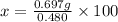
x = 145.21 g ≈ 145 g
145 grams of a 0.480 % KCN solution contains 697 mg of KCN.