Answer:
0.258 mg of iron remains.
Step-by-step explanation:
To solve this problem we can use the formula
M₂ = M₀ *
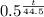
Where M₂ is the mass remaining, M₀ is the initial mass, and t is time in days.
Using the data given by the problem:
M₂ = 2.000 mg *
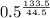
M₂ = 0.258 mg