Answer:
The empirical formula is:
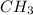
Step-by-step explanation:
In a sample of 100 g:
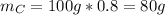
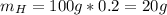
To determine the molecular formula you need to calculate the moles of each element. To do that the atomic weights are needed:
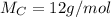
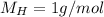
So, the moles of each one:
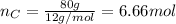
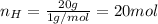
Now, you need to take this values to non-fractional numbers (keeping the ratio):
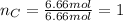
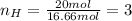
Therefore the empirical formula is:
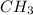
The molecular formula probably is a multiple of the empirical due to the carbon's valence.