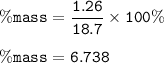The percentage by mass of ammonium fluoride in the solution : 6.738%
Further explanation
Given
18.7 g of NH4F solution
1.26 g NH4F
Required
The percentage by mass
Solution
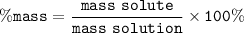
mass solute = mass Ammonium fluoride
mass solute = 1.26 g
mass solution = 18.7 g
Input the value :