Answer:
12 moles of H₂SO₄
Step-by-step explanation:
The reaction equation is given as;
3H₂SO₄ + 2Fe⁻ → Fe₂(SO₄)₃ + 3H₂
From the balanced reaction equation:
Number of moles of Fe = 8moles
So;
2 mole of Fe will react with 3 moles of H₂SO₄
8 moles of Fe will therefore yield
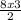 = 12 moles of H₂SO₄
= 12 moles of H₂SO₄