Answer:
PCl₅ = 223.4 torr
PCl₃ = 6.8 torr
Cl₂ = 26.4 torr
Step-by-step explanation:
For gas substances, the equilibrium constant can be calcultaed based on the partial pressures (Kp). For a generic reaction:
aA(g) + bB(g) ⇄ cC(g) + dD(g)
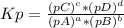 , where pX is the partial pressure of X.
, where pX is the partial pressure of X.
The reaction with the gas mixture given is:
PCl₅(g) ⇄ PCl₃(g) + Cl₂(g)
Kp = [(pPCl₃)*(pCl₂)]/(pPCl₅)
Kp = [13.2*13.2]/217
Kp = 0.803
When more Cl₂ is added, for Le Chatêlier's principle, the equilibrium will shift for the left, more PCl₅ will be formed, and the equilibrium will be reestablished.
The initial total pressure was 243.4 torr, so if it jumps to 263.0 torr, it was added 19.6 torr of Cl₂, so the partial pressure of Cl₂ is 32.8 torr. For the reaction:
PCl₅(g) ⇄ PCl₃(g) + Cl₂(g)
217.0 13.2 32.8 Initial
+x -x -x Reacts (stoichiometry is 1:1:1)
217 + x 13.2-x 32.8-x Equilibrium
So, the equilibrium constant must be:
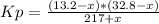
0.803 = (432.96 - 46x + x²)/(217 + x)
432.96 - 46x + x² = 174.251 + 0.803x
x² - 46.803x + 258.71 = 0
By Bhaskara's equation:
Δ = (46.803)² - 4*1*258.71
Δ = 1,155.68
x =[-(- 46.803) ±√1,155.68]/2
x' = (46.803 + 33.99)/2
x' = 40.40
x'' = (46.803 - 33.99)/2
x'' = 6.40
x < 32.8, so x = 6.40
The new partial pressures are:
PCl₅ = 217.0 + 6.40 = 223.4 torr
PCl₃ = 13.2 - 6.40 = 6.8 torr
Cl₂ = 32.8 - 6.40 = 26.4 torr